Trasis: 20 years of innovation at the service of nuclear medicine
3.5 million patients treated worldwide by 2023
A new building in Liège, 350 employees and a promising future.
Since it was founded in 2004 by Gauthier Philippart (CEO) and Jean-Luc Morelle (CTO), Trasis has set itself the ambitious goal of making new radiopharmaceuticals available to the medical community for the diagnosis of cancer and neurodegenerative diseases. At the same time, the company has emerged as a key player in theranostics, an innovative form of targeted therapy that is revolutionising cancer treatment.
In 20 years, many patients have benefited from Trasis innovations, including more than 3.5 million in 2023 alone. This achievement testifies to the company’s global impact on nuclear medicine.
Nuclear medicine: an area of activity with the wind in its sails
Nuclear medicine is defined as the use of radioactivity for diagnosis or treatment. This medical speciality uses radioactive substances, known as radiotracers or radiopharmaceuticals, which are chemical combinations of an active molecule and a radioactive isotope, resulting in a product that can be injected into the patient. Once administered into the body, these radiopharmaceuticals play an essential role, enabling specific targeting of the organs or tissues to be examined during medical imaging tests such as PET-Scan (Positron Emission Tomography). The same technology can also be used in cancer treatments involving radioisotopes specific to this application.
The future of nuclear medicine is promising, with technological advances improving the accuracy of diagnosis and care, and opening up new possibilities in medical research and personalised patient treatment.
Gauthier Philippart, co-founder and CEO of Trasis: “At Trasis, we are fully committed to realising a bold vision: a world where cancer is no longer synonymous with death. We firmly believe that nuclear medicine is an essential weapon in this fight. That’s why we’re striving to provide cutting-edge, ever more effective tools to turn this vision into reality. Our aim is clear: to offer healthcare professionals the most advanced means of diagnosing and treating cancer effectively, in order to improve patients’ prospects of survival and quality of life. At Trasis, we are driven by the conviction that every technological advance, every innovation, brings us one step closer to a future where cancer is beaten.”
For more than 20 years, Liège-based Trasis has been contributing to the development of nuclear medicine and is a world leader in the field of radiopharmaceuticals. Specialising in the supply of equipment, ingredients and innovative methods, it offers complete solutions for the development, industrial production and administration of all current and future radiopharmaceuticals. By 2023, nearly one in two patient doses worldwide had been manufactured or administered using Trasis solutions.
The key to success: a response at every stage in the life cycle of a radiopharmaceutical.
With the benefit of solid experience acquired over the years and the growing confidence of many players in the sector, Trasis has gradually extended its range of products and services to meet the evolving needs of its customers, including research centres, production companies and hospitals.
By listening carefully and taking a pragmatic approach to its market, Trasis has become the preferred contact for its partners, who rely on it to support them in their own developments.
“For us, innovation is the art of making the technically and economically possible in the service of health. The challenge is to develop simple, high-performance solutions for our customers that are also easily acceptable to health agencies. Innovation also means combining technologies from other fields, such as our investigations into smartphones as part of our dreams of miniaturisation. Our ambition is for nuclear medicine to be able to overcome a growing number of types of cancer“, emphasises Jean-Luc Morelle, co-founder and CTO of Trasis.
Trasis offers integrated, global solutions at every stage in the life cycle of a radiopharmaceutical, from development to administration to the patient. Thanks to Trasis, radiopharmaceuticals are developed more quickly, industrial production is more efficient, quality control is easier to carry out and administration is safer.
Trasis attaches great importance to the development of its human resources; its business requires a wide range of different expertise, necessary for the creation, production and monitoring of its products. The founders take great care to maintain a supportive working environment, placing well-being at work and empowerment at the heart of their concerns, seeing every member of staff as essential drivers of development and innovation.
Thanks to its family shareholding, Trasis can guarantee continuity in strategic decisions and a long-term commitment. The company’s profits enable it to finance its infrastructure, grow its team and gradually enhance its offering. This ongoing reinvestment in the development of Trasis bears witness to the founders’ determination to guarantee sustained, long-term growth.
The company’s flagship products include :
- AllinOne: a benchmark in radiopharmaceutical synthesis
AllinOne is Trasis’ best-seller, offering unrivalled flexibility in the synthesis of radiopharmaceuticals, compatible with a wide range of radioactive isotopes. Suitable for both research and industrial production, this equipment is a perfect illustration of Trasis’ capacity for innovation and its response to the complex needs of this constantly evolving market.

- QC1: innovation in radiopharmaceutical quality control
A champion of compactness, Trasis has achieved the feat of bringing together in a single instrument, QC1, all the quality controls that, until now, required around ten.
QC1 streamlines the quality control of radiopharmaceuticals by automating and accelerating their release, while reducing operator exposure to radioactivity. QC1 represents a significant step forward in laboratory efficiency, enabling them to deliver a growing variety of radiopharmaceuticals more quickly and with greater safety.
- Unidose: safe administration to patients
One of the first instruments developed by Trasis, Unidose automatically prepares individual radioactive doses for patients on demand, thereby protecting hospital staff. Setting new pharmaceutical standards and considerably simplifying the day-to-day work of nursing staff, Unidose has proved very popular, and is now used in most nuclear medicine departments in Belgium and France.
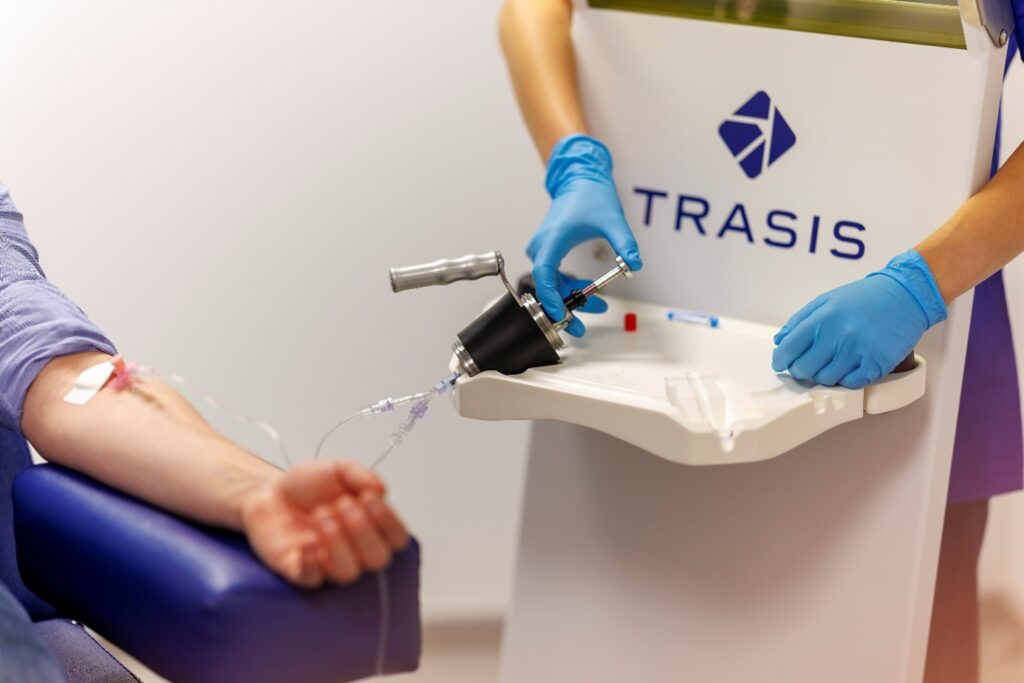
- GMP services: tailor-made solutions for pharmaceutical companies
Trasis works closely with major pharmaceutical groups on the development of innovative radiopharmaceutical molecules and drugs, such as the diagnosis and/or treatment of prostate cancer, Alzheimer’s and Parkinson’s diseases, as well as other specific forms of cancer and pathologies.
As a GMP (Good Manufacturing Practices) certified facility for the production of ingredients and medicines, Trasis develops, synthesises on its production lines, packages and sterilises raw materials for its own devices. The company has recently started making its production lines available to third parties. As part of its ongoing support for its partners, Trasis also assists them with regulatory affairs relating to medicines and health products.
Constantly evolving, Trasis is continually adapting its infrastructure and human resources to deliver its ever-expanding range of services.
A new 5,000 m² building and reinforced production lines
Trasis is entering a new phase in its development with a new building adding 5,000 m² to the existing 3,500 m². The new space houses a mechanical assembly workshop of almost 700 m², user-friendly office and community areas, synthesis and quality control laboratories, and clean rooms.
“With this brand new infrastructure, we have a pillar to support our transformation into a “factory of the future 4.0”. We are very enthusiastic about deploying our activities within this innovative framework, and the positive results are already being felt on a daily basis. We’re working on our efficiency, so that we can continue to innovate and perform while retaining our flexibility. We’re retaining the agility of an SME while meeting the demands of growth in terms of efficiency and organisation,” says Thomas Colmant, Head of Operations at Trasis.
This expansion marks an important milestone in the development of Trasis and its 20th anniversary, which will be celebrated at the inauguration on 18 April 2024.
This event is an opportunity to celebrate past successes and, above all, to look to the future, because Trasis is not stopping here. Its future is being built right now, with the acquisition of a 6-hectare site in the commune of Ans, paving the way for the next stage in its expansion. This move demonstrates Trasis’ ongoing commitment to remaining at the forefront of innovation, while further anchoring its local roots in Liège and Belgium.
More than 250 new jobs created since 2021
Trasis benefits from solid local expertise, with 92% of its workforce coming from the Liège region. Since 2021, the company has launched a massive recruitment drive, welcoming almost 250 new employees with a wide range of profiles to join the adventure. More than 350 employees are now taking part in the expansion of the company, which is aiming to employ 700 people by 2030.
A wide range of profiles, making up a multi-disciplinary team represented by some thirty different professions, ensure a fully in-house development and production chain for instruments and consumables. The company is currently looking for a wide range of profiles: sales support manager, field service engineer, sales administration officer, HSE specialist, mechanical production supervisor, production supervisor, radiochemistry researcher, etc.
Research and development occupies a prominent place at Trasis, accounting for almost a third of the current workforce, subdivided into complementary application areas such as chemical and mechanical sciences, IT and regulatory affairs. More than half of the staff are employed in operations, and their main task is to assemble the instruments and consumables in the cleanroom with meticulous attention to detail and dexterity. Finally, at Trasis, around thirty technicians travel the world to install and maintain the instruments marketed, and several people with dynamic profiles are responsible for commercial and structural activities.
The complementary nature of all these functions, combined with the values of empowerment and good humour that are so typical of Trasis, set it apart from its competitors and go a long way to explaining the company’s current success.
Think global, act local
With solutions deployed in nearly sixty countries around the world, Trasis remains firmly committed to maintaining close proximity to its customers. While the decision-making, development and production centre is based in Liège, since 2020 the company has established technical services and logistics teams in the United States and France, further reducing response and delivery times for its customers.
With 99% of its turnover coming from exports, Trasis was honoured with the EY “Scale up” award in 2018 and won the AWEX Wallonia Golden Export Award in 2022, recognising its success and contribution to Wallonia’s reputation abroad.
The company’s financial performance bears witness to its dynamism, with regular annual growth of 40%. Sales are set to rise from €25 million in 2020 to €70 million in 2023.
Thanks to a healthy operating profitability (EBIT) of 17%, Trasis is self-generating the resources it needs to finance the development of its infrastructure and new products, which represents more than €5 million in R&D alone. The family shareholders continuously reinvest profits in innovation and international expansion.
Trasis is also investing in the diversification of its activities, by supporting start-ups such as Abscint and Livedrop. It has also acquired one of its suppliers, Grenoble-based Eras Labo, strengthening its position in nuclear medicine and beyond.
About the Trasis story: from pioneer to leader in nuclear medicine
2004: The founding of Trasis
Driven by a spirit of innovation and vision, Jean-Luc Morelle and Gauthier Philippart founded Trasis in 2004 at “B3”, a small office in the ULiège chemistry buildings. Their aim? To develop revolutionary solutions in nuclear medicine.
2008: First major innovations
First put into service in 2008 at CHR Namur, the Unidose automatically prepares individual radioactive doses for patients on demand, thereby protecting hospital staff. Considerably simplifying the daily work of nursing staff, the Unidose is proving very popular. It is rapidly becoming standard equipment in most nuclear medicine departments in France.
2010-2012: towards universal radiosynthesis solutions.
In 2010, Trasis shook up the sector with its AllinOne synthesiser, a universal platform capable of synthesising any radiotracer for both research and routine production.
In 2012, Trasis unveiled its little brother, the mini AllinOne, primarily intended for the treatment of irradiated targets for radioelement producers and for the synthesis of radiopharmaceuticals labelled with radio-metals. Trasis is thus strengthening its position as technology leader in a new niche. Also in 2012, Trasis moved to the Ans site to the west of Liège, with 17 employees and 800 m2.
2014-2016: expanding services and infrastructure
Trasis’ commitment to its field and its market took concrete form with the development of a regulatory support service in 2014 and the commissioning of its first cleanroom in 2016, marking a turning point in its offering for the flexible production of small batches of ingredients for research.
2017-2023: growth and new frontiers
Trasis reaches 50 employees in 2017. The introduction of EasyOne in 2019, the smallest commercial synthesiser designed for hospitals, demonstrates Trasis’ ongoing commitment to its market. By 2022, the company already has 230 employees, demonstrating that its vision is aligned with the needs of its market. In 2023, Trasis launched its first QC1, a concentration of analytical devices in a single instrument designed for quality control of radiopharmaceutical products. It replaces around ten laboratory instruments and simplifies product control and release operations for industrial pharmacists.
2024: a new era of innovation and expansion
2024 is shaping up to be an emblematic year for Trasis, marked by the opening of its new buildings in Ans, a symbol of its ongoing commitment to excellence and innovation. These welcoming premises, designed to ensure the well-being and stimulation of our employees, embody the ambitions of Trasis as a key player in its field. With more than 350 talented employees on board, Trasis is consolidating its leadership position and strengthening its roots in Liège while expanding internationally.
True to its DNA, Trasis is constantly innovating. Numerous products are in the process of maturing, several of which are due to be launched in 2024 and 2025. Like its predecessors, they will help to make nuclear medicine more effective and accessible, so that as many patients as possible can benefit.
For more information: www.trasis.com
Press contact
Florence Delvaux
Press officer
+32 496 94 45 40
hello@florencedelvaux.com